Amazing Tips About Can Electrons Move Without Voltage

Electrical Fundamentals Ppt Download
Electrons on the Move
1. The Unseen Dance of Electrons
Ever wondered what those tiny electrons are up to inside, say, a copper wire when it's just sitting there, minding its own business? You might think they're patiently waiting for a jolt of voltage to send them scurrying along. But the truth is, these little guys are always in motion! It's like a microscopic mosh pit in there.
Think of it like this: imagine a room full of toddlers. Even without you telling them to run from one side to the other, they're still going to be bouncing around, bumping into things, and generally causing a delightful chaos. That's pretty much what electrons are doing, even when there's no voltage present. This is called "thermal motion" or "random motion."
The crucial part is, this motion is completely random. They're not going anywhere in particular. They're just jiggling and wiggling due to their temperature. The hotter the wire, the more vigorously they're bouncing around. Its a bit like a crowd at a concert; they're all moving, but not necessarily in a coordinated way.
So, can electrons move without voltage? Absolutely! They're constantly in motion, thanks to the magic of thermal energy. It's just that without voltage, there's no net flow of electrons in a specific direction. They're just doing their own thing, like tiny, energetic particles in a perpetual state of excitement.
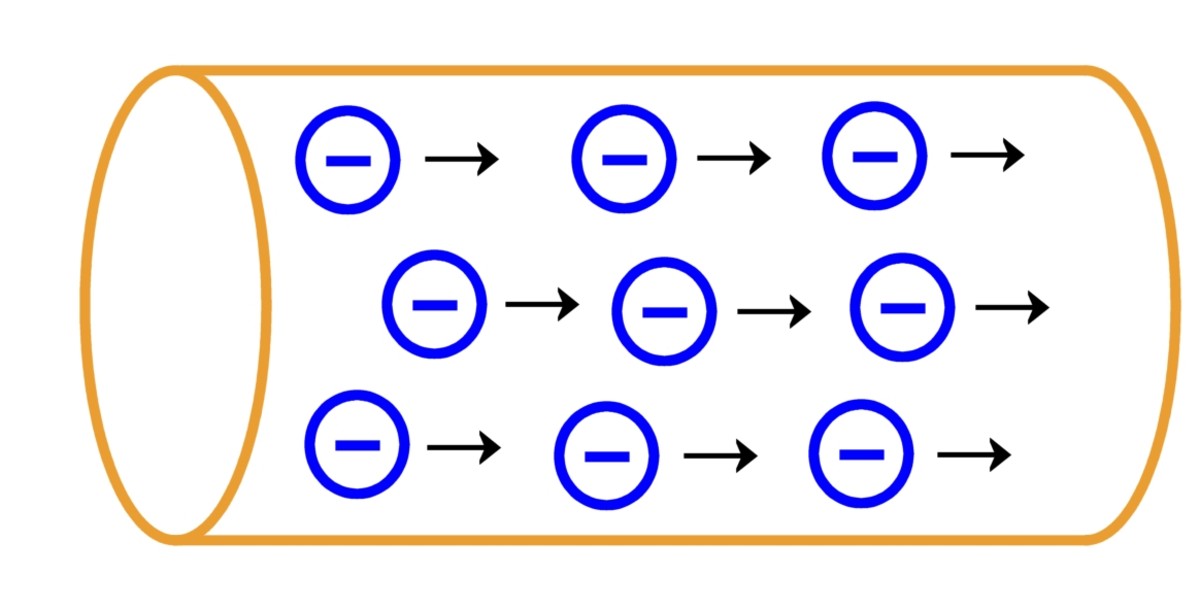
Drift Velocity
2. From Chaos to Order
Okay, so we know electrons are always buzzing around. But how does electricity actually flow? That's where voltage comes into play. Voltage is like a shepherd, gently nudging those wayward electrons in a particular direction. It's the force that transforms the random jiggling into a coordinated march.
When you apply a voltage to a wire, you create an electric field. This field exerts a force on the electrons, causing them to drift in a specific direction. This is what we call "drift velocity." Now, don't go thinking that electrons are suddenly zooming at lightning speed. Drift velocity is actually quite slow, often just a fraction of a millimeter per second. It's like a really, really slow-motion stampede.
Think about it like this: imagine our room full of toddlers again. If you start calling them all to come get candy on one side of the room, they'll still be bouncing around and bumping into each other. But now, there's a general movement towards the candy. That's drift velocity — a slow, deliberate drift superimposed on the existing random motion.
The amazing thing is that even though the individual electrons are moving slowly, the effect is almost instantaneous. It's like a chain reaction. One electron bumps into another, which bumps into another, and so on, transmitting the electrical signal incredibly quickly. So, while the electrons themselves aren't sprinting, the effect of their movement is what powers our devices.

METALS Bonds And Properties Alloys Pure Elements. Ppt Download
Conductors, Insulators, and Semiconductors
3. Where Electrons Roam Free (or Not)
Not all materials are created equal when it comes to electron movement. Some materials, like copper and silver, are excellent conductors, meaning electrons can move through them relatively easily. Others, like rubber and glass, are insulators, meaning they strongly resist the flow of electrons. And then there are semiconductors, like silicon, which fall somewhere in between, and their conductivity can be controlled.
In conductors, there are plenty of "free electrons" — electrons that aren't tightly bound to individual atoms and are free to roam around the material. This makes it easy for them to respond to an electric field and contribute to electrical current. Insulators, on the other hand, have very few free electrons. Their electrons are tightly bound to their atoms and are not easily dislodged.
Semiconductors are particularly interesting because their conductivity can be manipulated by adding impurities or applying an external field. This is what makes them so useful in electronic devices like transistors and integrated circuits. By controlling the flow of electrons in semiconductors, we can create complex circuits that perform all sorts of amazing tasks.
So, the ease with which electrons can move through a material depends on its atomic structure and the availability of free electrons. Conductors are like wide-open highways for electrons, insulators are like impenetrable walls, and semiconductors are like roads with adjustable speed limits.
+can+move+through+matter..jpg)
Unit 6 Electricity & Ppt Download
Temperature's Influence
4. The Thermal Tango of Electron Movement
Temperature plays a significant role in the movement of electrons. As we mentioned earlier, higher temperatures mean more vigorous random motion. But it also affects the drift velocity of electrons when voltage is applied.
In general, increasing the temperature of a conductor increases its resistance. This is because the more vigorously the atoms in the conductor are vibrating, the more frequently the electrons collide with them, hindering their flow. It's like trying to walk through a crowded room where everyone is randomly bumping into you.
In semiconductors, the effect of temperature is more complex. Increasing the temperature can increase the number of free electrons, which can increase conductivity. However, it can also increase the scattering of electrons, which can decrease conductivity. The overall effect depends on the specific semiconductor material and the temperature range.
So, temperature can be both a friend and a foe to electron movement. It provides the initial energy for random motion, but it can also hinder the orderly flow of electrons when voltage is applied. It's all about finding the right balance to optimize the performance of electronic devices.
Electrons and Quantum Mechanics
5. The Weird World of Electron Behavior
If you really want to understand electron movement, you have to delve into the realm of quantum mechanics. At the atomic level, electrons don't behave like tiny billiard balls. Instead, they behave like waves, and their behavior is governed by the laws of quantum mechanics.
Quantum mechanics tells us that electrons can only exist in certain energy levels, or "quantum states." When an electron absorbs energy, it can jump to a higher energy level. When it releases energy, it can fall to a lower energy level. These transitions between energy levels are responsible for many of the phenomena we observe in the world around us, including the emission of light from light bulbs and the absorption of light by plants.
Furthermore, the wave-like nature of electrons means that they can tunnel through barriers that they classically shouldn't be able to penetrate. This phenomenon, called "quantum tunneling," is used in many electronic devices, such as flash memory and tunnel diodes.
So, while we can often get away with thinking of electrons as tiny particles, their true nature is far more complex and fascinating. Quantum mechanics provides the deepest and most accurate understanding of their behavior.

Ch 16.1 Electric Charge And Static Electricity Ppt Download
FAQ
6. Your Burning Questions Answered!
Still got questions? Here are some common queries about electrons and voltage:
Q: Do electrons ever stop moving?
A: Not really! Even at extremely low temperatures, electrons retain some residual motion due to quantum mechanical effects. It's like they're perpetually buzzing with a tiny amount of energy.
Q: Is it dangerous to touch a live wire?
A: Absolutely! A live wire has a significant voltage difference relative to your body. If you touch it, you provide a path for electrons to flow through you, which can cause serious injury or even death. Always exercise extreme caution when working with electricity.
Q: What's the difference between current and voltage?
A: Voltage is the "push" that drives electrons through a circuit. Current is the rate at which electrons are flowing. Think of voltage as the water pressure in a pipe and current as the amount of water flowing through the pipe per second.
Q: Can I create electricity from just heat?
A: Yes, you can! This is the principle behind thermoelectric generators. They use the Seebeck effect, where a temperature difference between two dissimilar conductors creates a voltage. However, the efficiency of thermoelectric generators is typically quite low.
Q: Why doesn't the wire get hotter when electrons are moving through it?
A: Actually, it does get hotter! The movement of electrons through a wire causes some energy to be dissipated as heat due to resistance. This is why light bulbs get hot and why wires can overheat if they carry too much current. The amount of heating depends on the current and the resistance of the wire, following Joule's law.